
Bohr Model Aluminium Atom Electron Structure Stock Vector (Royalty Free
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
aluminium bohr s model clipart 10 free Cliparts Download images on
What is the Bohr model for aluminum? A Visual Depiction: A Bohr model is a way of visually depicting the structure of an atom of a particular element. An atom is the main component of an.

Introducir 47+ imagen modelo bohr aluminio Abzlocal.mx
Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr's model was taken seriously, despite the many assumptions that Bohr needed to derive it. Figure \(\PageIndex{1}\): Quantum numbers and energy levels in a hydrogen atom.
Aluminum (Al) AMERICAN ELEMENTS
Category: Science & Tech Key People: Niels Bohr Related Topics: atom See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.

Lewis structure Electron Aluminium Periodic table Bohr model, aluminum
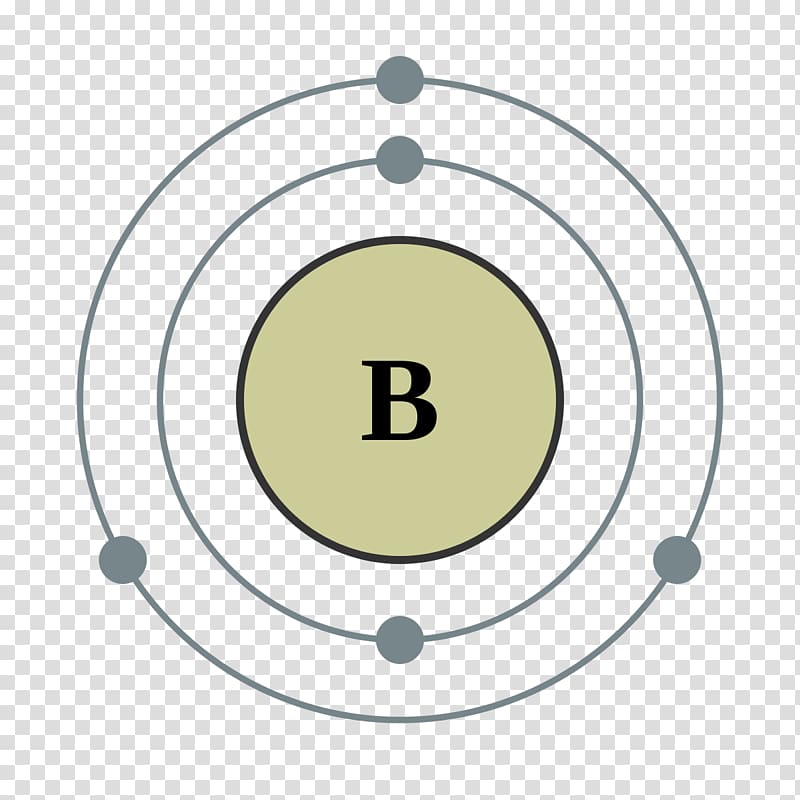
Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number.

Atomic structure of aluminum Brainly.in
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.
3d model of aluminum atom 3d render of atom structure of aluminum
9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.

Aluminium bohr s model 10 free HQ online Puzzle Games on
1 λ = − ℜ( 1 n2 2 − 1 n2 1) Except for the negative sign, this is the same equation that Rydberg obtained experimentally. The negative sign in Equation 2.6.5 and Equation 2.6.6 indicates that energy is released as the electron moves from orbit n2 to orbit n1 because orbit n2 is at a higher energy than orbit n1.
Bohr model diagram of Aluminium Al in atomic physics Stock Vector
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

aluminum model Cheaper Than Retail Price> Buy Clothing, Accessories and
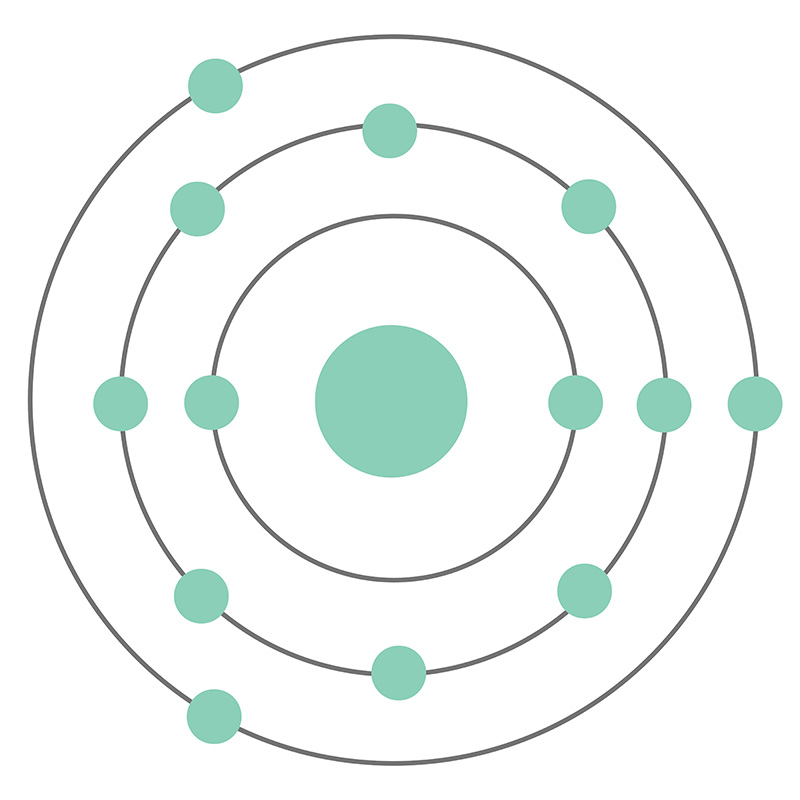
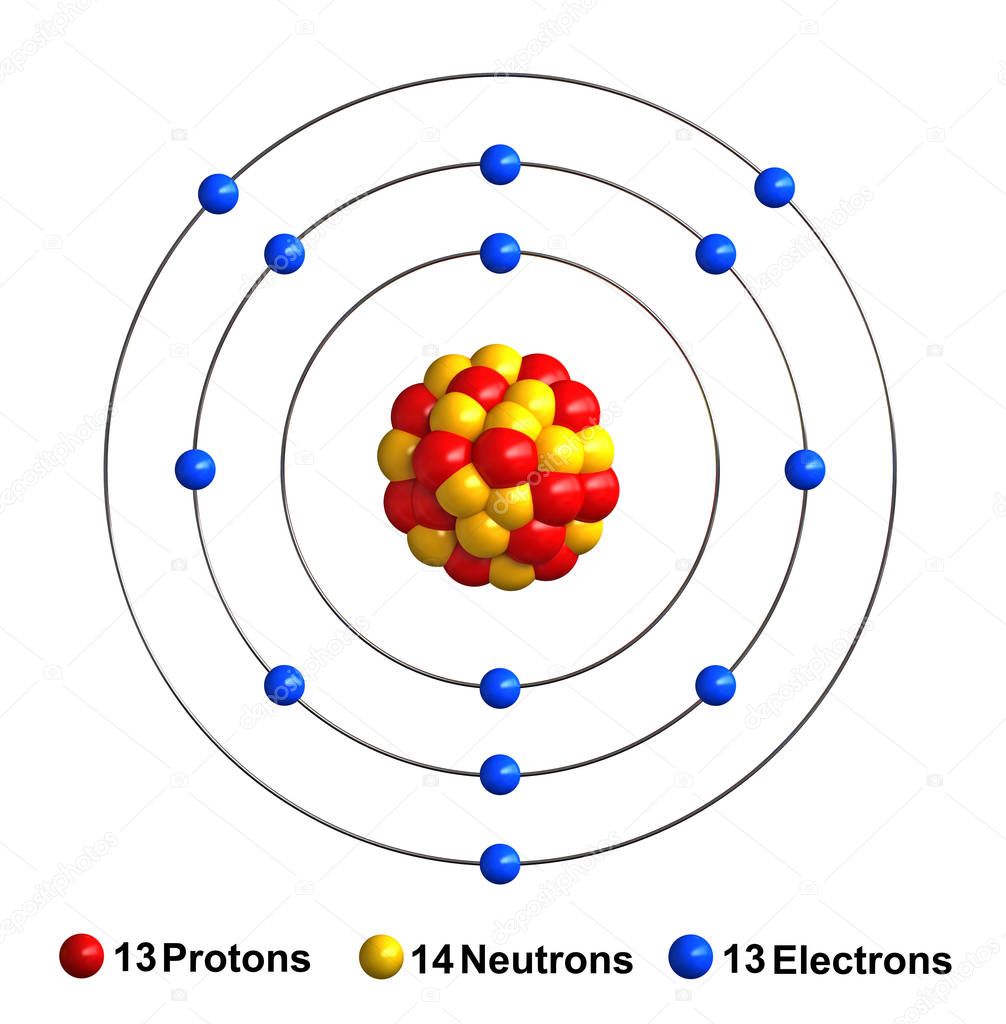
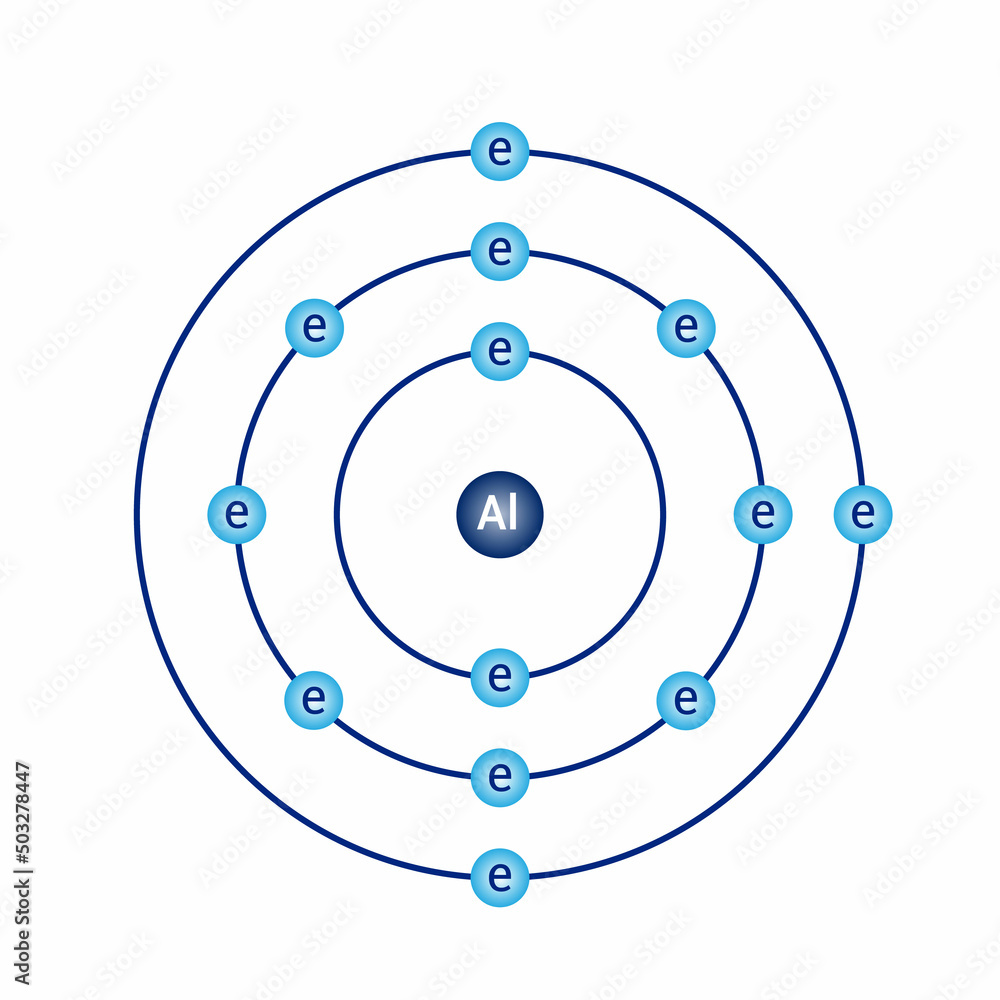
The Bohr Model of Aluminum (Al) has a nucleus with 14 neutrons and 13 protons. This nucleus is surrounded by three electron shells. The first shell of the Bohr diagram of Aluminum has 2 electrons, the 2nd shell has 8, and the 3rd shell has 3 electrons. Also check - How to draw Bohr model diagram for an atom?
aluminium bohr model clipart 10 free Cliparts Download images on
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.

Atomic Structure (Bohr Model) for Aluminum (Al) YouTube
Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out: http://www.chemistnate.com
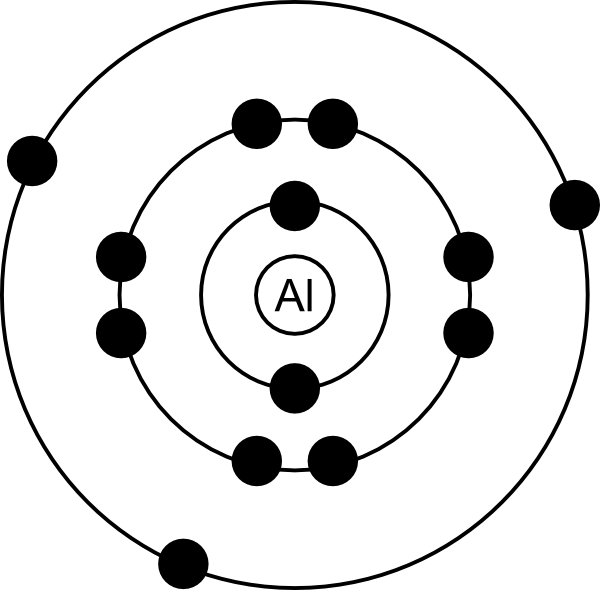
Aluminum Bohr Model ClipArt Best
In this video we'll look at the atomic structure and Bohr model for the Aluminum atom (Al). We'll use a Bohr diagram to visually represent where the electron.
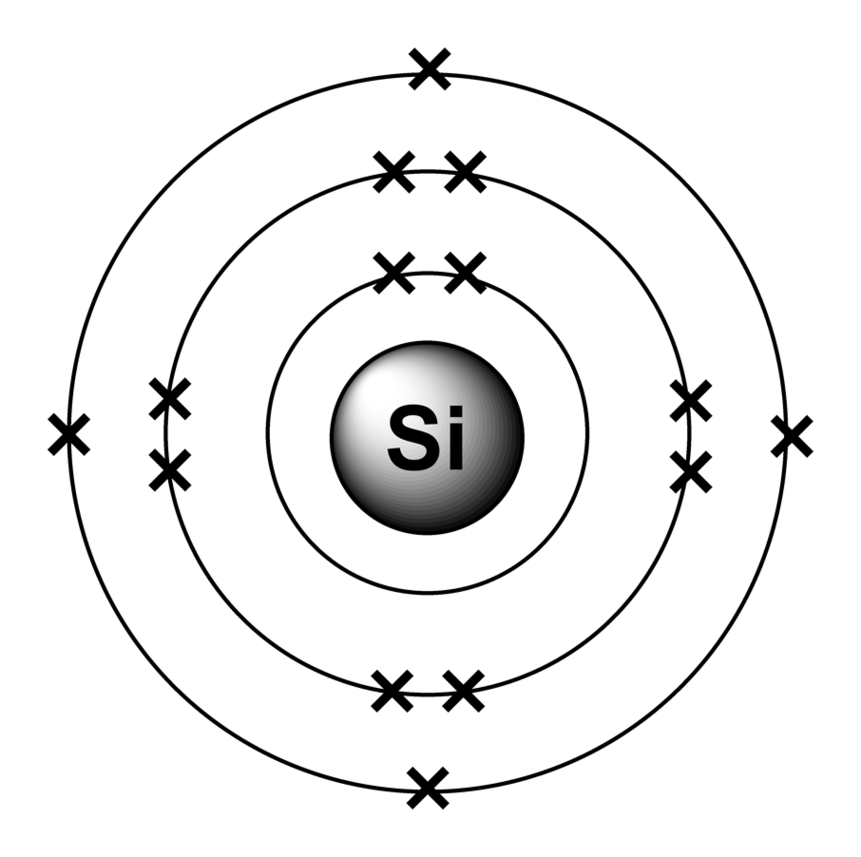
Aluminum Bohr Model ClipArt Best
The Aluminum Bohr Model is a comprehensive atom model that has become a cornerstone of modern chemistry. Developed in 1913 by Danish physicist Niels Bohr, this model is based on the idea that electrons orbit the nucleus of an atom in distinct layers or shells.

Aluminum Bohr Model
The Bohr Model of Aluminum (Al) has a nucleus that contains 14 neutrons and 13 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Aluminum contains 3 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Aluminum (Al)?
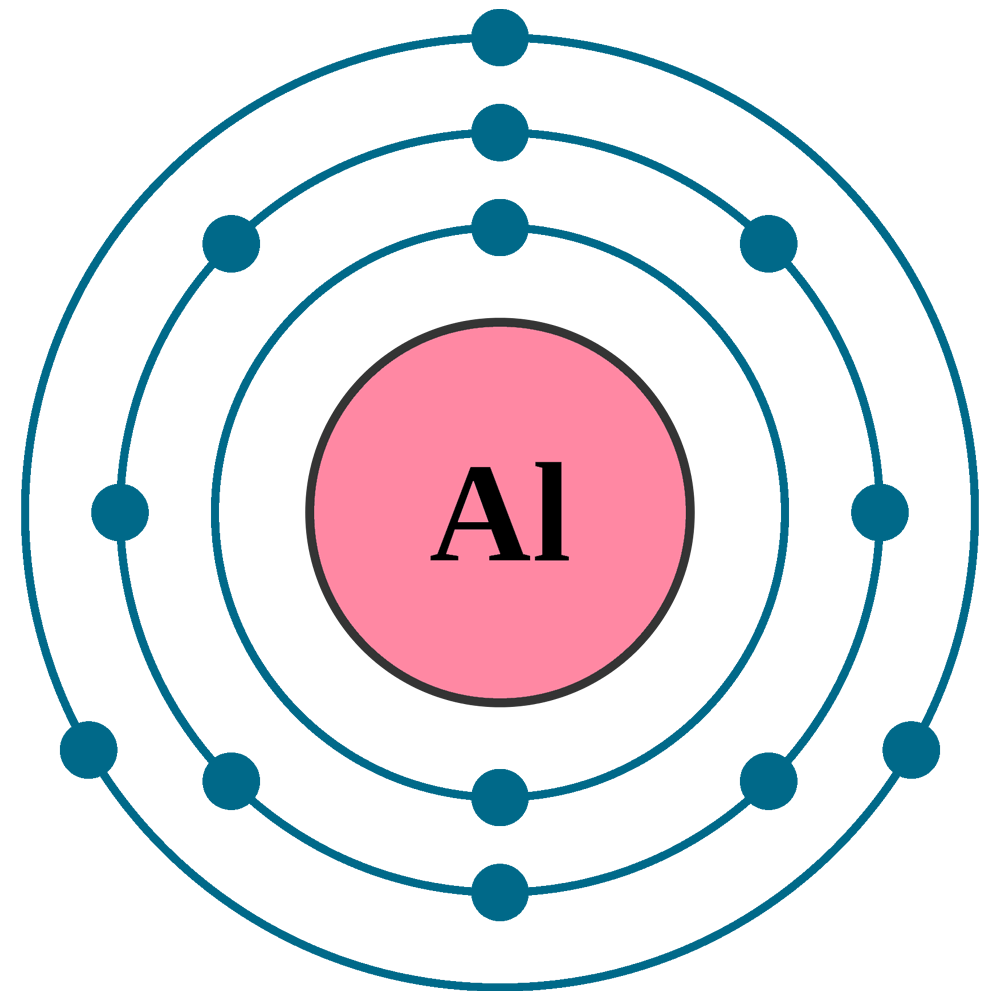
Bohr Model of Aluminum PNG Free Download PNG Arts
Atomic & Molecular Structure How to Make a Model of an Aluminum Atom for Students ••• Updated April 24, 2017 By Renee Claire An atom is a unit of matter that includes a dense central nucleus surrounded by negatively charged electrons.